The ions are then held in place by their very strong ionic bonds. The above structure is known as a crystal lattice, and sodium chloride—like most ionic compounds—is a crystalline solid. You will learn more about this in future lessons on the different types of solids.
Anode material for lithium ion battery-Tycorun Batteries
The metal cations lose electrons to the nonmetal anions so they “stick” together in an ionic compound. This means that ionic bonds are formed by the attraction of these two oppositely charged particles. A covalent bond is formed between nonmetal atoms. The nonmetals are connected by a shared pair of valence electrons.

Source Image: fanaticalfuturist.com
Download Image
Ionic compounds conduct an electric current when melted or dissolved in water. Figure 4.7.5 4.7. 5: In an ionic solution, the A+ ions migrate toward the negative electrode, while the B− ions migrate toward the positive electrode. Example 4.7.1 4.7. 1. Write the dissociation equation of solid NaCl in water.
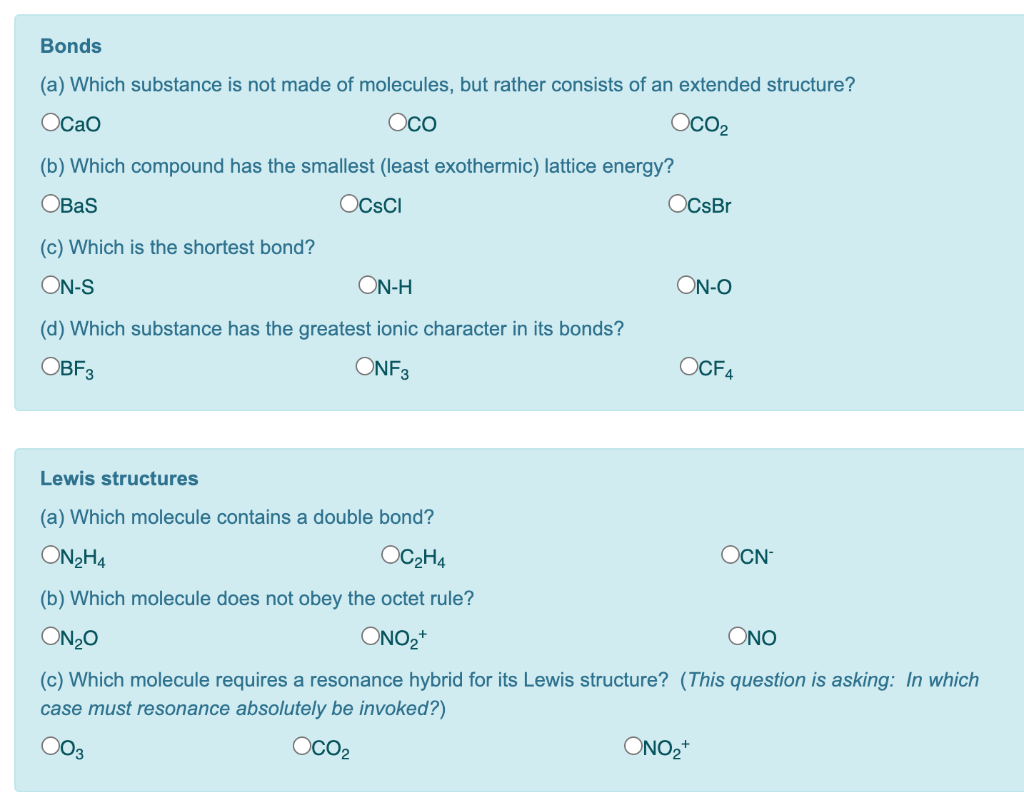
Source Image: chegg.com
Download Image
Ion Photos and Images & Pictures | Shutterstock
AboutTranscript. To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. Then, identify the anion and write down its symbol and charge. Finally, combine the two ions to form an electrically neutral compound. In this video, we’ll walk through this process for the ionic compound calcium bromide.

Source Image: facebook.com
Download Image
If A Substance Is Ionic Then It Likely Will
AboutTranscript. To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. Then, identify the anion and write down its symbol and charge. Finally, combine the two ions to form an electrically neutral compound. In this video, we’ll walk through this process for the ionic compound calcium bromide.
Ionic bonds form when the charges between the metal cation and non-metal anion are equal and opposite. This means that two Cl−1 anions will balance with one Ca+2 cation. This makes the formula for calcium chloride CaCl2. For the example Aluminum Oxide Al2O3. Aluminum has an oxidation state of +3 or Al+3.
Ionic Bonds In an ionic bond,… – Biology Physics Chemistry | Facebook
When electrons are transferred and ions form, ionic bonds result. Ionic bonds are electrostatic forces of attraction, that is, the attractive forces experienced between objects of opposite electrical charge (in this case, cations and anions). When electrons are “shared” and molecules form, covalent bonds result.
How to tell if compound is Ionic or Covalent? – All concepts

Source Image: topblogtenz.com
Download Image
Ionic liquids-metal surface interactions: Effect of alkyl chain length on coordination capabilities and orientations – ScienceDirect
When electrons are transferred and ions form, ionic bonds result. Ionic bonds are electrostatic forces of attraction, that is, the attractive forces experienced between objects of opposite electrical charge (in this case, cations and anions). When electrons are “shared” and molecules form, covalent bonds result.

Source Image: sciencedirect.com
Download Image
Anode material for lithium ion battery-Tycorun Batteries
The ions are then held in place by their very strong ionic bonds. The above structure is known as a crystal lattice, and sodium chloride—like most ionic compounds—is a crystalline solid. You will learn more about this in future lessons on the different types of solids.

Source Image: tycorun.com
Download Image
Ion Photos and Images & Pictures | Shutterstock
Ionic compounds conduct an electric current when melted or dissolved in water. Figure 4.7.5 4.7. 5: In an ionic solution, the A+ ions migrate toward the negative electrode, while the B− ions migrate toward the positive electrode. Example 4.7.1 4.7. 1. Write the dissociation equation of solid NaCl in water.

Source Image: shutterstock.com
Download Image
There is Something in the Water – Life in the Lab
Ionic compounds generally form from metals and nonmetals. Compounds that do not contain ions, but instead consist of atoms bonded tightly together in molecules (uncharged groups of atoms that behave as a single unit), are called covalent compounds. Covalent compounds usually form from two or more nonmetals.

Source Image: thermofisher.com
Download Image
SOLVED: If a substance is ionic, then it likely will what ?
AboutTranscript. To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. Then, identify the anion and write down its symbol and charge. Finally, combine the two ions to form an electrically neutral compound. In this video, we’ll walk through this process for the ionic compound calcium bromide.

Source Image: numerade.com
Download Image
15 What is an ion? ideas | electrons, periodic table, solutions
Ionic bonds form when the charges between the metal cation and non-metal anion are equal and opposite. This means that two Cl−1 anions will balance with one Ca+2 cation. This makes the formula for calcium chloride CaCl2. For the example Aluminum Oxide Al2O3. Aluminum has an oxidation state of +3 or Al+3.

Source Image: pinterest.com
Download Image
Ionic liquids-metal surface interactions: Effect of alkyl chain length on coordination capabilities and orientations – ScienceDirect
15 What is an ion? ideas | electrons, periodic table, solutions
The metal cations lose electrons to the nonmetal anions so they “stick” together in an ionic compound. This means that ionic bonds are formed by the attraction of these two oppositely charged particles. A covalent bond is formed between nonmetal atoms. The nonmetals are connected by a shared pair of valence electrons.
Ion Photos and Images & Pictures | Shutterstock SOLVED: If a substance is ionic, then it likely will what ?
Ionic compounds generally form from metals and nonmetals. Compounds that do not contain ions, but instead consist of atoms bonded tightly together in molecules (uncharged groups of atoms that behave as a single unit), are called covalent compounds. Covalent compounds usually form from two or more nonmetals.